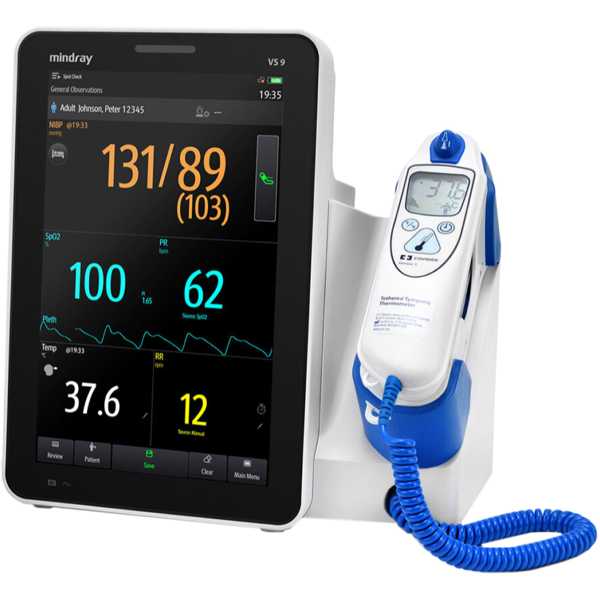
Mindray VS 9

Device Model:
VS-9
Manufacturer:
Shenzhen Mindray Bio-Medical Electronics Co. Ltd., Mindray Building, Keji 12th Road South, High-tech Industrial Park, Nanshan, Shenzhen 518057, CHINA.
Measuring functions:
Blood pressure, Arrhythmias, SpO2, Temperature, ECG, Respiration
Primary Client Use:
Intended for patient monitoring
Measurement Site:
BP & SpO2: Finger
Measurement Occurrence:
BP & SpO2: Continuous measurement
Connected Health Technologies:
USB
Availability:
Available Currently
Description:
The Mindray VS 9 is a vital signs monitor. Its blood pressure measurement technology has been proven to be accurate, with a 2-star Medaval rating and information regarding the accuracy of its oxygen saturation measurement technology does not appear to be available. Blood pressure measurements and oxygen saturation measurements are taken from the finger. It is intended for bedside patient monitoring.
Assessment:
The technology used in the Mindray VS 9, to measure blood pressure, has passed in a clinical validation study, in a general population, according to a recognised standard protocol, as published in a peer-reviewed publication. There appears to be no peer-reviewed clinical validation information available on the technology used in the Mindray VS 9 to measure peripheral oxygen saturation.
Recommendations:
| Accuracy Assessment | Recommendation | Basis | |
| BP | Medaval | ★★ Recommendation | Recent clinical validation; recent protocol |
Validation Publications:
Guo N, Zhang Y, Chen W, Zhong H, Li L, Xie H, Zhu W, Liu J, Li S. Validation of the Mindray VS9 Vital Signs Monitor in a combined adult and pediatric population according to ISO Standard 81060-2:2018. Blood Press Monit. Epub: 2024 Mar 25. doi: 10.1097/MBP.0000000000000704. PMID: 38523458.
81060-2:2019 - Pass General population (Note: Inflation method)
81060-2:2019 - Pass General population (Note: Deflation method)